Change Registration Information for a Facility
July, 2016
Table of Contents
- Select Facility
- Review Facility Information
- Update Options
- View Your Registration and Listing Information
- Enter PIN/PCN Information
- Complete Updates
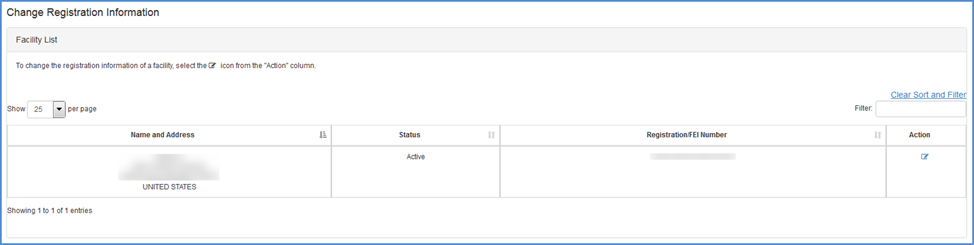
Select Facility
Select the facility you would like to update by clicking the
 icon from the "Action" column.
icon from the "Action" column.
Note: You may only update one facility at a time.
Facilities Listing Screen
Review Facility Information
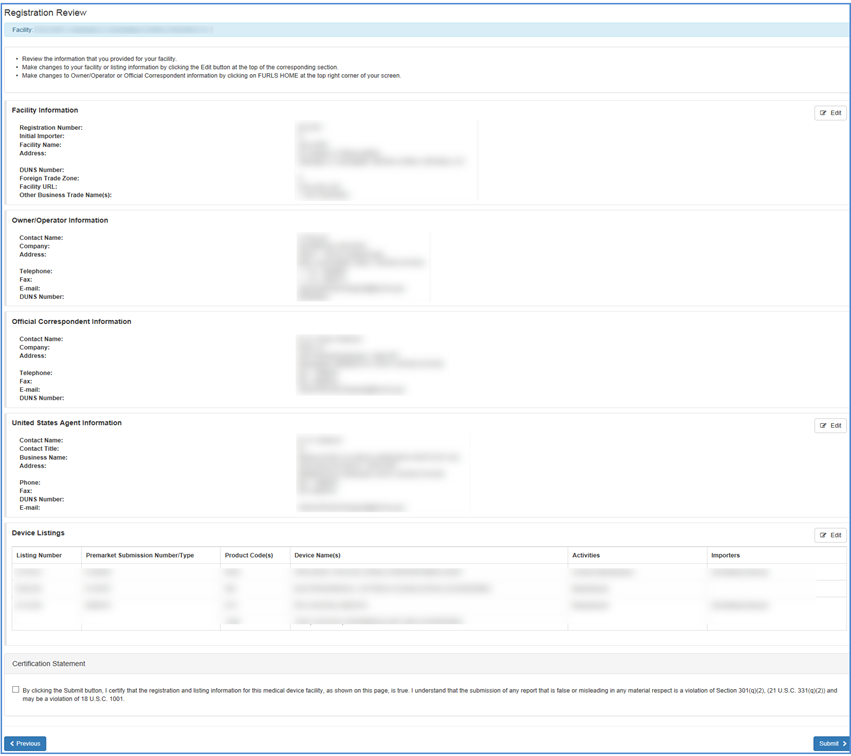
In the Review Registration Information Screen you may view all information on record for the selected facility including:
- Facility name and location.
- DUNS Number
- Phone and fax numbers
- Facility URL
- Trade name(s)
- Owner/Operator information
- Official Correspondent information
- United States Agent information (for foreign facilities only)
- Medical devices associated with the facility. You can choose to edit any of the facility or device listing information.
Review Registration Information Screen
Update Options
From the Review Registration Information screen you may:
- Edit information about the facility
- Edit U.S. agent information (foreign facilities only)
- Review
all information associated with a device
- Edit information for a Listing: Remove a product from facility’s listing
- Edit selected listing
- Add new products
These options are used to update facility information only. To update Owner/Operator or Official Correspondent information, go to the accounts management site.
Edit Facility Information
If you choose to edit facility information, a screen displaying all current information on record will appear.
Carefully review all information to ensure that it is correct. You may change any incorrect or outdated facility information by highlighting and typing over text.
| Table 1 - Facility Name and Address Data Description | |
| Form Field *mandatory | Description of Information Needed |
| *Facility Name | The name of the specific facility being registered. This should be differentiated from any other facilities owned by the company. |
| Address Line 1 | The physical location of the
company (example: a street address). Rural locations can use other physical/geographic identification (example: RR565). |
| Address Line 2 | Additional space is provided for additional address information. |
| *Zip or Postal Code | Domestic addresses: type zip code.
Foreign addresses: type postal code. Note: If no codes are used, type "NONE." |
| *City | The name of the city where the facility is located. |
| *State/Province/Territory | U.S. Domestic registrations select
a state from the pull-down list. Foreign registrations, click on the words, "Choose a Province/Territory" and select the applicable province. |
|
*Phone Number - country code - 3-digit area/city code - 7-digit phone number - extension |
Domestic addresses: fields for
three-digit area code and seven-digit phone number are displayed. Enter
numbers only with no dashes or other special characters. The extension to be dialed
(if any) is optional, but recommended. Foreign addresses: the country code, the three-digit city code, and the phone number are mandatory. |
|
FAX Number - Country code - 3-digit area/city code - 7-digit phone number |
The number of the FAX machine used
at the facility. Domestic addresses: type area code and phone number. Foreign addresses: type country code, area code, and phone number. |
| DUNS Number | The unique numeric identifier assigned to your business entity by Dun & Bradstreet. |
| Foreign Trade Zone (only for a domestic facility) | Check this box if your facility is located in a foreign trade zone |
| Facility URL | The URL for the web page that references the specific facility being registered. |
| Other Business Trade Name(s) | Other trade names that are used to identify the facility. |
| Initial Importer | Select the Yes radio button if you are an initial importer. |
Facility Location Information Screen
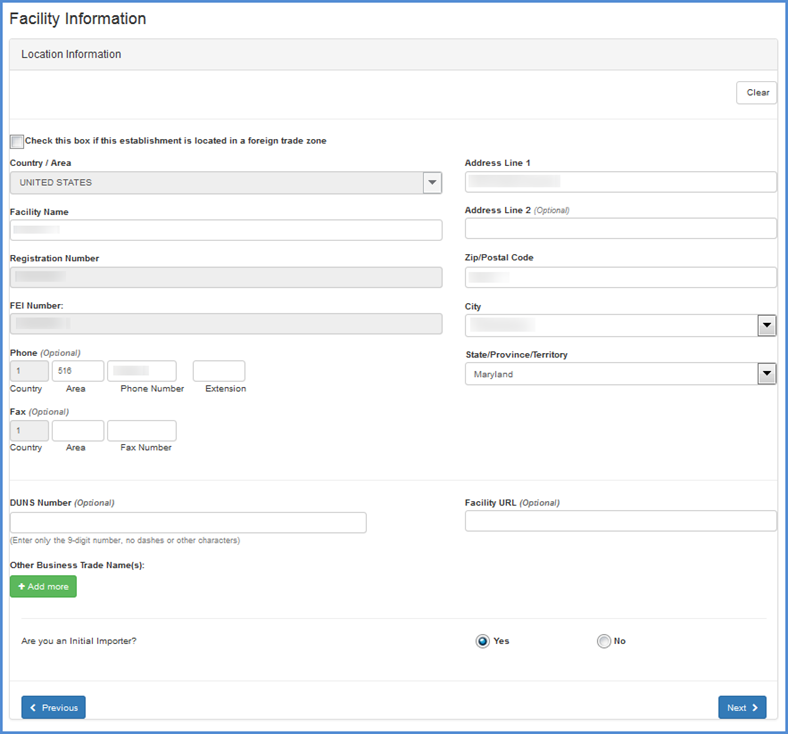
Once you have completed the fields on this screen, click "Next" to be navigated to the Review Registration Information Screen.
Update U.S. Agent Information (Foreign registrations only)
By clicking on the "Edit" button, you may update name, location, or contact information for the U.S. Agent by highlighting and typing over the text you want to change. You may also add content in fields that were previously empty.
U.S. Agent Review and Edit Section

Once you have finished completing the fields on this screen, the Facility and Device Information Screen will be displayed again. Choose "Submit" to save the changes.
Review Device Listing Information
By selecting the "Edit" button next to Device Listings in the Registration Review page, you may review information about any device listing before proceeding to update any information.
All recorded information for the device listings will be displayed, including, listing number, premarket submission number/type, product code(s), device name(s), activities, proprietary names, and importers.
This option is used to update facility information only. To update Owner/Operator or Official Correspondent information, go to the accounts management site.
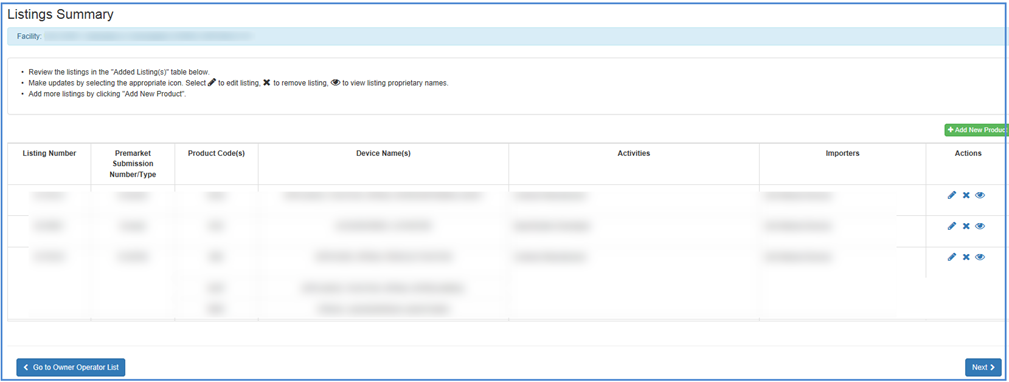
Review Listing Information
View Your Registration and Listing Information
After selecting the Facility
Registration that you wish to update, all recorded information may first be
reviewed before making any changes.
You must choose to either: 1) View your registered facilities or 2) View your
Device Listings.
Click on the View Your Registered Facilities option. The system will display information on all of your registered facilities including the registration status, status reason and facility name and address, as well as the payment status and expiration date.
You may select a registration and then choose View Selected Registration to view additional information such as the owner/operator and official correspondent information.
Choose View Your Device Listings for
a view of all listing numbers. Make updates by selecting the appropriate icon.
Select  icon to edit listing,
icon to edit listing,
 icon to remove listing,
icon to remove listing,
 icon to view listing proprietary names.
icon to view listing proprietary names.
All recorded information for the device will be displayed, including product codes, product names, registration numbers, registration status, and reason, as well as the activities that are associated with the device listing.
Once you have reviewed the device information, select "Next" to be returned to the Registration Review screen.
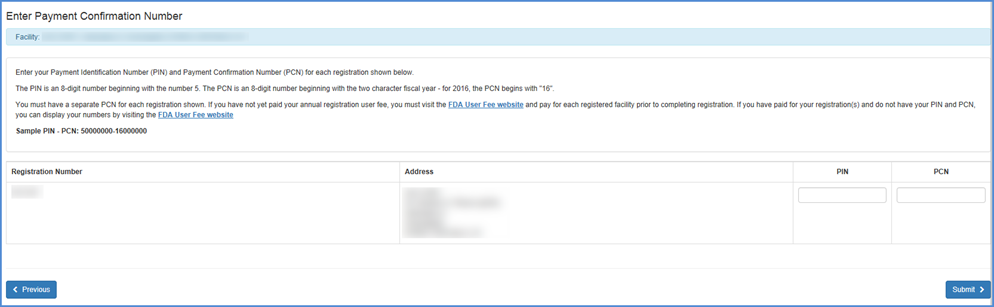
Enter PIN/PCN Information
Please make sure to enter your PIN/PCN information. If you do not yet have a PIN/PCN, please visit the FDA User Fee website to complete this process.
Complete Updates
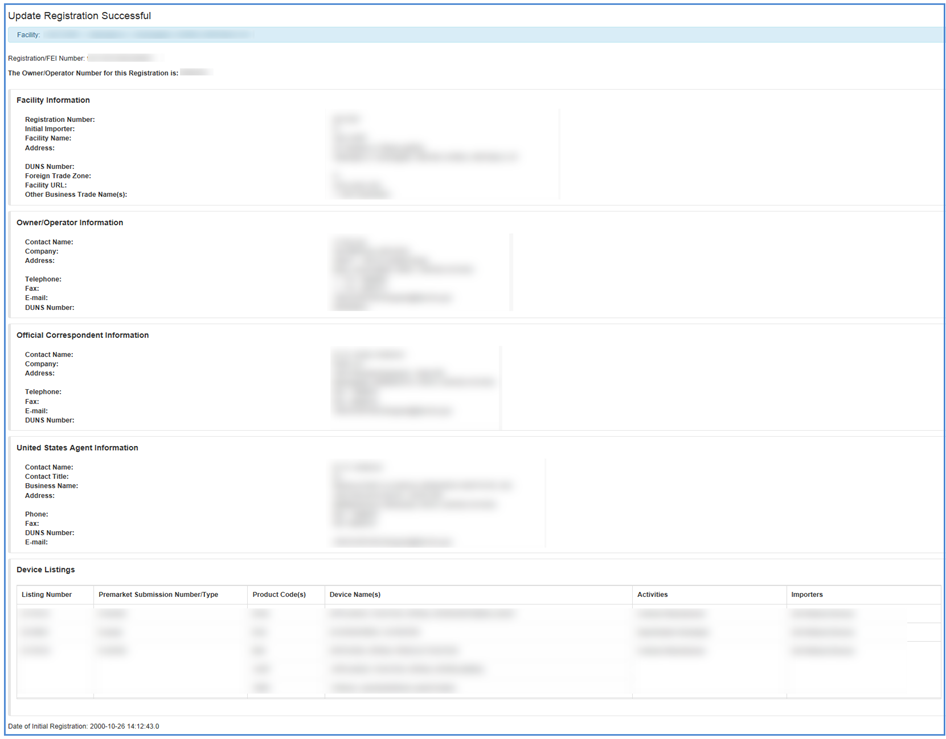
Once you have completed all necessary updates for the facility and/or identified medical devices, click the box by the certification statement at the bottom of the Facility and Device Information Screen and click "Submit."
You will receive a confirmation of your successfully updated registration information with all current information for the facility displayed.